Integrative Medicine Case Reports, Volume 4, Issue 1 (January), 2023
Protocol for impact of mindfulness meditation on health-related quality of life in hematopoietic cell transplantation recipients
KEY WORDS |
ABSTRACT |
|
Mindfulness-based meditation
|
Background: The procedure of Hematopoietic cell transplantation (HCT) is associated with a significant risk of morbidity and mortality and, consequently, significant distress in the HCT recipients. Few mindfulness-based interventions (MBI) in HCT recipients had heterogeneous intervention practices and equally heterogeneous tools to measure outcomes. While preliminary studies showed the feasibility and usefulness of MBI, subsequent randomized controlled trials were flawed by low intervention uptake and no differences in outcomes. We propose a simple and acceptable MBI using an app that may ensure better adherence. Summary: We propose a randomized controlled trial (RCT) where HCT recipients in the intervention arm will be initiated into awareness using ‘Inner engineering’ online and practice self-guided mindfulness meditation (Isha Kriya) using an app. The outcomes will measure the global and subdomain (physical, social/family, functional, emotional, and BMT-specific) quality of life (QoL) using validated HCT questionnaires such as the Functional Assessment of Cancer Therapy – Bone Marrow Transplantation (FACT-BMT) and Patient-Reported Outcomes Measurement Information System – Global Health (Adult version) (PROMIS-GH). Key massage: The primary endpoint would be a change in the BMT-specific subdomain of the FACT-BMT questionnaire and the secondary endpoint would be a change in the FACT-BMT total scores and global health scores and adherence to MBI. Should the study endpoints be met, the practicality and usefulness of MBI in the HCT setting will be confirmed in this study. doi: 10.38205/imcr.040129 |
|
*Corresponding Author:
|
Background
Hematopoietic cell transplantation (HCT) is a major curative intent, the standard of care procedure for patients with several hematological disorders, including malignancies. However, several short and long-term adverse events include non-relapse mortality of 20–30% associated with the procedure. There is enough data that the health-related quality of life (HR-QoL) is impaired after HCT. This is from the treatment related toxicity, infections, and graft versus host disease (1). Previous studies have reported that there is a significant increase (22–43%) in the distress level in HCT survivors (2). This includes fear of disease recurrence, memory loss, loneliness, and somatic concerns, irrespective of age, gender, or time since HCT (2). This distress may negatively impact recovery, immune reconstitution, graft vs. host disease, and mortality (3). Only a few mindfulness-based interventions (MBI) have been done in the HCT setting to manage distress in HCT recipients. These studies have heterogeneous intervention practices and equally heterogeneous tools to measure outcomes (Table 1).
MBI methods
There are several ways to deliver mindfulness meditation and/or relaxation techniques, as evident from the following studies in the HCT setting. Initial preliminary studies focused on the feasibility of delivering mindfulness meditation using guided one-on-one structured meditation sessions continuously throughout the HCT period (4,5). Subsequently, randomized controlled trials (RCT) used a self-administered stress management program alone or combined with exercise training offered at specific time points after HCT (6,7).
MBI outcome methods
The pilot studies used several tools to record the effect of interventions like the visual analog scale or scales for anxiety, depression, fatigue, etc. (4,5). The subsequent RCTs used validated and standardized Questionnaires: European Organization for Research & Treatment of Cancer QoL-C30 (EORTC QLQ-C30), and Study Short Form 36 (SF-36) questionnaires (6,7).
Impact of MBI
The preliminary pilot studies did show an improvement in symptoms after the intervention. These studies also showed feasibility and acceptance in small sample cohorts (4,5). The RCTs with larger sample sizes had low intervention uptake and consequently failed to differentiate the QoL outcomes using MBI (6,7). These studies show that the MBI has to be simple and acceptable to the patients. It is best offered in one-one sessions starting before the HCT and continued throughout the HCT period to adhere to the MBI. Also noteworthy is that the outcomes should precisely measure anxiety, fatigue, or mental health rather than only QoL measures for an effect to be seen. Few meta-analyses have shown that MBI reduced fatigue, anxiety, and depression in patients with cancer (8,9).
The proposed study will focus on using a culturally acceptable (yogic) way of awareness initiation (Inner Engineering Online) and self-guided mindfulness meditation (Isha Kriya) as an app for routine practice. We hypothesize that a culturally acceptable self-guided meditation method as an app will have higher acceptance and adherence and possibly more significantly improve QoL and reduce stress on short-term follow-up after HCT. Though several studies show that Isha Kriya reduces stress levels and improves immunity in healthy volunteers and other disorders by modifying the immune system (10), there is no data on applying these techniques in recipients undergoing HCT who report high levels of distress. The Functional Assessment of Cancer Therapy – Bone Marrow Transplantation (FACT-BMT) is a self-administered questionnaire, which is used to assess the QoL in patients receiving bone marrow transplantation. It combines the FACT-General (G) assessment tool, which assesses physical well-being, social/family well-being, emotional well-being, and functional well-being, along with Bone Marrow Transplantation Sub-scale (BMTS), which measures the BMT-specific concerns (11). The Patient-Reported Outcomes Measurement Information System–Global Health scale (PROMIS-GH) is a tool that compiles patients’ perceptions of physical (GPH) and mental (GMH) health (12). Another recent study is assessing the effect of mobile health application-based cognitive behavioral therapy on post-traumatic stress disorder and PROMIS measures in cancer survivors (13).
Table 1: Summary of studies on mindfulness-based interventions in the HCT settings
| Author, Center, Year | Sample size | Method/intervention | Method/assessment | Results | Impact |
| Bauer-Wu, Atlanta, 2008(4) | 20, single arm | 17-minute guided meditation CD, one-one MM sessions starting at apheresis, weekly, Before HCT, week1, discharge, day30, day+100 | Visual analogue scales (VAS)—for comfort, anxiety, mood, and pain-before and after each MM session, Symptom Experience Scale (SES)
Hospital Anxiety and Depression Scale (HADS) |
Improvements in symptoms were observed, 80% compliance | Feasibility and preliminary support of a mindfulness meditation intervention |
| Grossman, Switzerland, 2014(5) | 64 randomized two arms | Mindfulness-based intervention (MBI) structured 8-week program compared to psycho-oncological telephone consultation, >6 months after HCT | Profile of HR-QoLin Chronic Disorders (PQoLC),
Functional Assessment of Cancer Therapy Scale (FACT-G), Center for Epidemiological Studies Depression Scale (CES-D); FACIT-F fatigue scale Spielberger Trait Anxiety Scale (STAI). |
Improvement in HR-QoL, reduction in anxiety and depression was observed after the MBI intervention; the benefits were modest after three months, Compliance >90% | MBI benefitted by improving the HR-QoL and overall well being of the patients, increased acceptance or feasibility, satisfaction and adherence. |
| Jacobsen, BMT-CTN, 2014 (6) | 711, randomized to 4 arms | Exercise and stress management training, paced abdominal breathing, progressive muscle relaxation technique with guided imagery, and coping self-statements, combination of exercise and training of stress management, usual care | Cancer and Treatment Distress (CTXD)
Study Short Form36 (SF-36), Pittsburgh Sleep Quality Index (PSQI) |
No variations in the Medical Outcomes, distress, or sleep summary scales for the physical and mental components. | Brief training for self-directed exercise or stress management was not effective |
| Braamse, Netherlands, 2016 (7) | 95 randomized to two arms | Stepped up care watchful waiting, Internet based self-help intervention, and in-person counseling/psychopharmacological treatment/referral versus usual care @ 13, 30, and 42 weeks | Hospital Anxiety and Depression Scale (HADS),
European Organization for Research & Treatment of Cancer Quality of LifeQuestionnaire-C30 (EORTC QLQ-C30), Patient Health Questionnaire-9 (PHQ-9), Spielberger State-Trait Anxiety Inventory: state version (STAI-state), Social Problem Solving Inventory-Revised (SPSI-R), Dutch General Self-efficacy Scale (DGSS) |
No variations in psychological distress or physical functioning | Low intervention uptake |
Aim
To evaluate the impact of Inner Engineering Online & Isha Kriya on HR-QoL and emotional domains in HCT recipients
Objectives (endpoints)
Primary
Change in BMT-specific subscale domain of the FACT-BMT questionnaire [Timeline baseline – 3 months after HCT]
Secondary
(1) Change in HR-QoL as assessed by FACT-BMT (version 4) questionnaire
(2) Change in HR-QoL in Global Mental Health as assessed by PROMIS® Scale v1.2 – (Global Health questionnaire) [Timeline baseline – 3 months after HCT]
(3) Adherence to the meditation practice as assessed by self-diary and weekly reminders
– [Timeline baseline – 3 months after HCT]
Exploratory
(1) Change in HR-QoL at 6/12-months after HCT
Study population
Inclusion criteria
(1) all adults (Age ≥ 18 years at the time of transplant),
(2) all transplant recipients (autologous/allogeneic)
(3 those willing to adhere to the app-based meditation practice for the period of the study
Exclusion criteria
(1) Those not willing to consent
(2) Those without access to a smartphone
(3) Those practicing meditation techniques of their own choice
Intervention
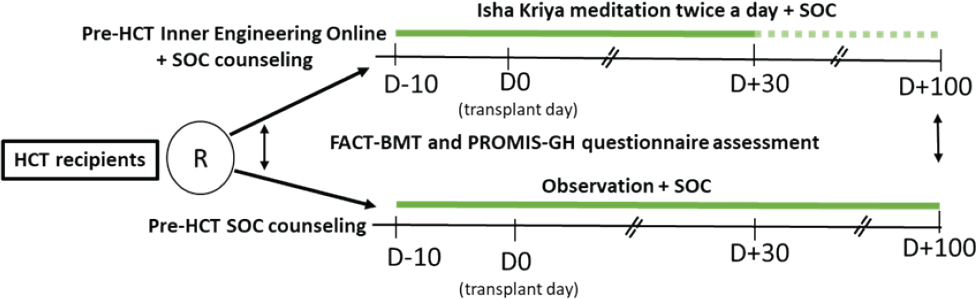
Institutional Human Ethics committee approval will be obtained before commencing the study. All participants will be included only after obtaining written informed consent. After screening and registration, recipients will be randomized to either standard of care (SOC) or SOC + Inner Engineering Online course followed by Isha Kriya meditation available on the Sadhguru app. The app is freely available on Play Store and Apple stores. The Inner Engineering Online course consists of seven 90-minute sessions that impart potent tools from the ancient science of Yoga, including Isha Kriya’s basis in several languages. It’s a guided meditation application with instructions available in the form of video and written manual in several languages. The guided meditation itself is 12 minutes in duration. It is recommended to be performed twice a day. The aim of Isha Kriya’s is to connect an individual with existence, and to channelise life as per their wish and vision. The regular practice helps achieve improved health, dynamism, peace, and overall well-being (14). Those recipients randomized to the intervention arm will have to complete the Inner Engineering online course before being admitted to the BMT unit for HCT, followed by the practice of Isha Kriya twice daily for the period of the HCT from day-10 to day + 30 post HCT. The practice of Isha Kriya beyond day + 30 till day + 100 will be at the discretion of the recipients and encouraged at least once daily (Figure 1). The QoL questionnaires PROMIS® Scale v1.2 – Global Health (mental + physical) and FACT-BMT (version 4) questionnaire are validated in Indian languages and routine use in our HCT recipients. These will be self-administered at baseline before the intervention and three months after the HCT.
Study design
open-label, single-center, randomized controlled trial

Figure 1: Study Schema showing randomization and outcome measures
Statistical analysis
Sample size calculation
Sample size calculation is based on the following assumptions. Difference between the means of experimental and control group of 0.1 with a standard deviation of 0.15 (5), probability (power) 0.8, type I error probability of 0.05. To reject the null hypothesis (H0) that the population means of the intervention group and control groups are equivalent, we will include 36 subjects in the intervention group and 36 in the control group. With an estimated auto + allo-HCT non-relapse mortality of 15% and an attrition rate of 15, we will need to recruit an additional 12 recipients in each arm.
Statistical analysis plan (SAP)
The analysis of endpoints will be done on an intention to treat basis. Domain and summary scores will be calculated for each instrument as recommended by the developers. The change in QoL scores will be compared between the two arms using the Kruskal-Wallis test. Baseline demographic and transplant characteristics will be compared using the chi-square test (χ2). A two-tailed p-value <0.05 will be considered for statistical significance.
Conclusion
Should the endpoints of the study be met, meaning there are statistical and clinically significant differences between the endpoints between the intervention and control arms, the usefulness of the MBI will be proven.
Authors’ contribution
All authors contributed to the manuscript design, write-up and approval of the final manuscript.
Source of funding
There is no funding for this manuscript.
Conflict of interest
All the authors have no relevant financial or non-financial interests to disclose.
Received Date: 05-02-22; Revised Date: 13-10-22
Accepted Date: 13-01-23
References
1. Bevans M, El-Jawahri A, Tierney DK, Wiener L, Wood WA, Hoodin F, et al. National Institutes of Health Hematopoietic Cell Transplantation Late Effects Initiative: The Patient-Centered Outcomes Working Group Report. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2017;23(4):538-51.
2. Sun C-L, Francisco L, Baker KS, Weisdorf DJ, Forman SJ, Bhatia S. Adverse psychological outcomes in long-term survivors of hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study (BMTSS). Blood. 2011;118(17):4723-31.
3. Syrjala KL, Langer SL, Abrams JR, Storer B, Sanders JE, Flowers ME, et al. Recovery and long-term function after hematopoietic cell transplantation for leukemia or lymphoma. JAMA. 2004;291(19):2335-43.
4. Bauer-Wu S, Sullivan AM, Rosenbaum E, Ott MJ, Powell M, McLoughlin M, et al. Facing the challenges of hematopoietic stem cell transplantation with mindfulness meditation: a pilot study. Integr Cancer Ther. 2008;7(2):62-9.
5. Grossman P, Zwahlen D, Halter JP, Passweg JR, Steiner C, Kiss A. A mindfulness-based program for improving quality of life among hematopoietic stem cell transplantation survivors: feasibility and preliminary findings. Support Care Cancer. 2015;23(4):1105-12.
6. Jacobsen PB, Le-Rademacher J, Jim H, Syrjala K, Wingard JR, Logan B, et al. Exercise and Stress Management Training Prior to Hematopoietic Cell Transplantation: Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0902. Biol Blood Marrow Transplant. 2014;20(10):1530-6.
7. Braamse AMJ, van Meijel B, Visser OJ, Boenink AD, Cuijpers P, Eeltink CE, et al. A randomized clinical trial on the effectiveness of an intervention to treat psychological distress and improve quality of life after autologous stem cell transplantation. Ann Hematol. 2016;95(1):105-14.
8. Duong N, Davis H, Robinson PD, Oberoi S, Cataudella D, Culos-Reed SN, et al. Mind and body practices for fatigue reduction in patients with cancer and hematopoietic stem cell transplant recipients: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;120:210-6.
9. Oberoi S, Yang J, Woodgate RL, Niraula S, Banerji S, Israels SJ, et al. Association of Mindfulness-Based Interventions With Anxiety Severity in Adults With Cancer: A Systematic Review and Meta-analysis. JAMA Network Open. 2020;3(8):e2012598-e.
10. Chandran V, Bermúdez M-L, Koka M, Chandran B, Pawale D, Vishnubhotla R, et al. Large-scale genomic study reveals robust activation of the immune system following advanced Inner Engineering meditation retreat. Proceedings of the National Academy of Sciences. 2021;118(51):e2110455118.
11. McQuellon RP, Russell GB, Cella DF, Craven BL, Brady M, Bonomi A, et al. Quality of life measurement in bone marrow transplantation: development of the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) scale. Bone Marrow Transplant. 1997;19(4):357-68.
12. Shaw BE, Syrjala KL, Onstad LE, Chow EJ, Flowers ME, Jim H, et al. PROMIS measures can be used to assess symptoms and function in long-term hematopoietic cell transplantation survivors. Cancer. 2018;124(4):841-9.
13. Smith SK, Somers TJ, Kuhn E, Laber E, Sung AD, Syrjala KL, et al. A SMART approach to optimizing delivery of an mHealth intervention among cancer survivors with posttraumatic stress symptoms. Contemporary Clinical Trials. 2021;110:106569.
14. https://isha.sadhguru.org/in/en/yoga-meditation/yoga-program-for-beginners/isha-kriya-meditation accessed January 09, 2023.