Integrative Medicine Case Reports, Volume 4, Issue 1 (January), 2023
Single-cell dissection of the obesity-exercise axis in adipose-muscle tissues implies a critical role for mesenchymal stem cells: Cell Metab. 2022 Oct 4;34(10):1578–1593.e6
ABSTRACT |
||
*Corresponding Author:
|
In this research, Yang et al. have done single cell analysis of obesity and exercise interactions to determine the response of mesenchymal stem cells (MSCs) white adipose and skeletal muscle tissue in mice models. The study concluded that, exercise and obesity have reverse effects on extracellular matrix (ECM) and genes involved in circadian rhythms in MSCs. doi: 10.38205/imcr.040133 |
Introduction
Since 1980, The number of people suffering from overweight and obesity has doubled and Now, approximately one third of the global population is categorised as overweight or obese (1). Obesity pandemic has an harmful effect on the overall physical and mental health and quality of life of an individual (2). Obesity is a multifaceted nutritional illness characterized by an increase in body fat mass as a result of an energy imbalance in the body. Physical exercise is often among the first therapeutic options recommended for obese people but exercise interventions have low compliance rates as compared to other interventions in humans (3).
Exercise has been demonstrated to help people lose weight, improve their metabolic health, and lower their chance of acquiring obesity-related disorders such as cardiovascular disease in many studies. The findings by these authors emphasize the importance of physical activity in alleviating obesity (4). It has been shown that exercise acts as an effective stimulus of Inguinal white adipose tissue (iWAT) remodeling primarily through changes in extracellular matrix (ECM), vascularization, and innervation (5).
Exercise can benefit body functions by modifying the expression of fundamental genes and proteins. Many studies have used mouse models to investigate various molecular mechanisms underlying the health benefits of exercise interventions on different diseases in humans.
This study provides a high-quality single-cell atlas of obesity exercise interactions in subcutaneous and visceral white adipose tissue and skeletal muscle in mice. It also explored the role of mesenchymal stem cells in obesity and exercise training responses in these three tissues.
This study aimed at identifying the molecular signatures in the cells mediating the systemic effects of exercise vs obesity in various tissues at single cell level and tissue level by utilizing most advanced techniques in single-cell technology and computational biology.
Study design
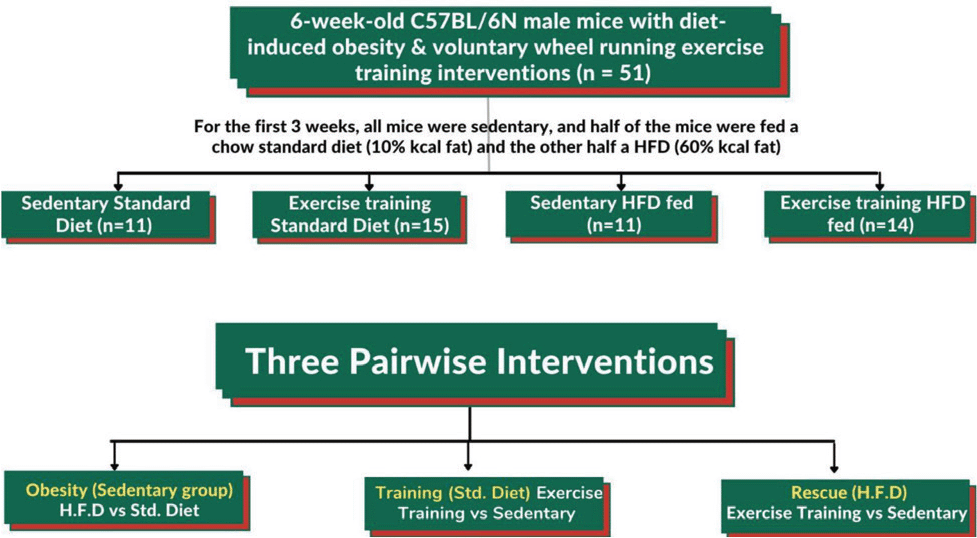
All the experiments had due approvals from the Animal Care and Use institutional Committee at Joslin Diabetes Centre in Boston, MA. C57BL/6N mice (n = 60) were procured from the Charles River Laboratories and were provided either a standard chow diet (10% kcal fat) or a high-fat diet (HFD) (60% kcal fat). The mice were kept in cages containing a running wheel for exercise training. The exclusion criteria was applied on the mice that ran less than 3 km/day (n = 9). The procedure and various interventions applied throughout the analysis of study is represented in Fig. 1. Perigonadal visceral (vWAT) and inguinal subcutaneous white adipose tissue (scWAT) and triceps muscle were dissected quickly. These tissues were either snap-frozen for use in future or processed straightaway to make a cell suspension for further processing.

Figure 1: Overview of the procedure and interventions
The molecular responses to exercise vs obesity, and the function of MSCs in both tissue-specific and multi-tissue level were determined using the methods listed below.
• Bulk mRNA sequencing
• Single-cell RNA sequencing
• Histological Analysis
• FACS-based cell isolation, RNA isolation, and quantitative PCR
• RNAscope LS Multiplex Fluorescent ISH assay
• Immunohistochemistry assay
Result
The researchers discovered that all three tissues have opposing responses while comparing exercising and non-exercising animals. The following conclusions were derived from this study:
• Seven distinct fibro-adipogenic progenitors (FAP) states were found in Skeletal muscles (SkM) and a single population consisting of Sca1 FAPs was confirmed as potential fibrogenic progenitors with the help of a single cell atlas.
• Pathway analysis at single cell level depicted the gene expression of circadian rhythm and extra-cellular matrix (ECM) remodeling in all three tissues being modulated by exercise and obesity in MSCs. In MSCs, Exercise was found to downregulate the ECM-related pathways while obesity upregulated them. It also implies that obesity and exercise may modify the fibro-inflammatory phenotype of interstitial progenitor cells (IPCs) by influencing the expression of ECM-related genes.
• The large human cohort analysis done by the researchers showed a negative relationship between the expression of DBP in scWAT and BMI and HOMA-IR. Hence, in mice and humans, training-induced circadian rhythm genes may act as regulators for the differentiation of MSC, influencing the metabolic activities of the whole body.
• The Intra-tissue analysis suggested that exercise could enhance anti-inflammatory and being effects by switching from RANKL-OPG to RANKL-RANK interaction, which is responsible for re-polarization of macrophages into an M2 state or recruitment of M2 macrophages in vWAT.
• The Inter-tissue study evaluation of the response to exercise training and obesity revealed directed ligand-receptor interactions between different immune cells of vWAT and SkM FAPs. MIF-CD74 signaling was decreased in M1 macrophages and DCs, on contrarily, slightly increased its interaction with M2 macrophages in the same tissue due to exercise training. They also hypothesized that intra (RANKL) and inter-tissue (MIF) signals regulate M2 macrophages and MIF released from SkM that further acts differently on various myeloid subtypes in vWAT.
Limitations
• The study was done only on mouse models and not on humans.
• The study also did not include additional physiological parameters like sex, age and different training types.
• The single-cell protocol was enhanced for cell types in stromal vascular fraction (SVF) only which led to limited results in parenchymal cell types.
• Proteomics analysis in blood and in vivo tracer techniques could also have been done to fully understand the cross-tissue communication.
Implication
This study is very informative as it enables us to determine the effect of exercise on obesity using MSCs as a biomarker at the cellular level instead of using plasma. The above study provided evidence that exercise, as a phenomenon, induces tissue-specific changes in the context of obesity. This study is also a first step towards deciphering those molecular mechanisms and different types of cells that moderate the comprehensive effects of exercise on obesity in various tissues. In summary, MSCs play a functional role in the three tissues in response to either obesity or exercise training, which is mediated by changes in their potential for fibrogenesis, inflammation, and differentiation.
Hence, it will be interesting to explore the potential of exercise-induced changes in MSCs in other diseases like cancer and neurodegeneration through more comprehensive cellular level-based studies. More future studies are also needed to understand better the role of MSCs in adipogenesis and how they can be used clinically for obesity.
Received Date: 23-12-23; Revised Date: 13-01-23
Accepted Date: 17-01-23
References
1. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. New England Journal of Medicine, 2017. 377(1): p. 13-27.
2. Wyatt, S.B., K.P. Winters, and P.M. Dubbert, Overweight and Obesity: Prevalence, Consequences, and Causes of a Growing Public Health Problem. The American Journal of the Medical Sciences, 2006. 331(4): p. 166-174.
3. Kirwan, J.P., J. Sacks, and S. Nieuwoudt, The essential role of exercise in the management of type 2 diabetes. Cleveland Clinic Journal of Medicine, 2017. 84(7 suppl 1): p. S15-S21.
4. Jakicic, J.M., et al., Role of Physical Activity and Exercise in Treating Patients with Overweight and Obesity. Clinical Chemistry, 2018. 64(1): p. 99-107.
5. Nigro, P., et al., Exercise Training Remodels Inguinal White Adipose Tissue Through Adaptations in Innervation, Vascularization and the Extracellular Matrix. 2022, Cold Spring Harbor Laboratory.