Integrative Medicine Case Reports, Volume 5, Issue 1 (January), 2024
Exercise reprograms the inflammatory landscape of multiple stem cell compartments during mammalian aging
KEY WORDS |
ABSTRACT |
|
Exercise
|
Engaging in exercise has the remarkable capacity to revitalize stem cells and enhance tissue revitalization in aging mice. This research delved into the cellular and molecular alterations occurring in a range of stem cell varieties post-exercise. It involved the elderly and young mice with aerobic exercise and analysing their stem cells at the transcriptomic level. The study demonstrated that exercise mitigates the heightened inflammation associated with aging while enhancing communication between cells, particularly via immune cells. Furthermore, it exerts a significant influence on the composition and gene expression of immune cells in both the bloodstream and tissues. In summary, this investigation provides a comprehensive insight into how exercise affects various types of aging stem cells and their surrounding environments.
doi: 10.38205/imcr.050146 |
|
Corresponding Author:
|
Introduction
A key aspect of aging is the diminished capacity of somatic stem cells to generate vital differentiated cells needed for tissue maintenance and repair in various organs including the brain, blood, muscle, fat, skin, and lungs (1). As the mouse brain ages, quiescent neural stem cells (qNSCs) in the hippo campus rise in diversity, alongside a decline in progenitor cells including activated neural stem cells (aNSCs) and neuroblasts in both the subventricular zone (SVZ) and hippocampus (2–5). Aging leads to a decrease in the quantity of skeletal muscle stem cells (MuSCs) and greater variability in their dormant state and response when activated. In older mice MuSCs are more susceptible to senescence, cell death, and loss of their ability to generate muscle tissue.
On the contrary, aging in old mice increases the quantity of hematopoietic stem cells (HSCs). Despite this numerical rise, the functionality of aged HSCs declines, and their specialization shifts toward the myeloid lineage. Analyses using single-cell RNA sequencing and proteomics indicate that changes in the stem cell environment in aging animals, such as immune cell infiltration and alterations in the extracellular matrix, may impact stem cell function. This emphasizes the necessity for a thorough exploration of stem cells and their surrounding niche to enhance our comprehension of the aging process in stem cells. Physical exercise alleviates age-related metabolic and immunological changes, thereby reducing the mortality risks associated with cardiovascular diseases and cancer. A growing body of research suggests that engaging in physical activity has a positive influence on the functioning of stem cells and the rejuvenation of tissues in aging animals. It enhances neurogenesis and improves learning in older mice, maintains the generation of new neurons in the hippocampus as animals grow older, and enhances muscle regeneration by increasing cyclin D1 expression in muscle stem cells (MuSCs). Furthermore, exercise results in an augmentation of common lymphoid progenitors in the bone marrow of aging mice, while not impacting the frequency of hematopoietic stem cells (HSCs) (6).
This study utilized a comprehensive single-cell atlas to explore the impact of exercise on the function of stem cells and tissue balance as organisms age.
Study Design
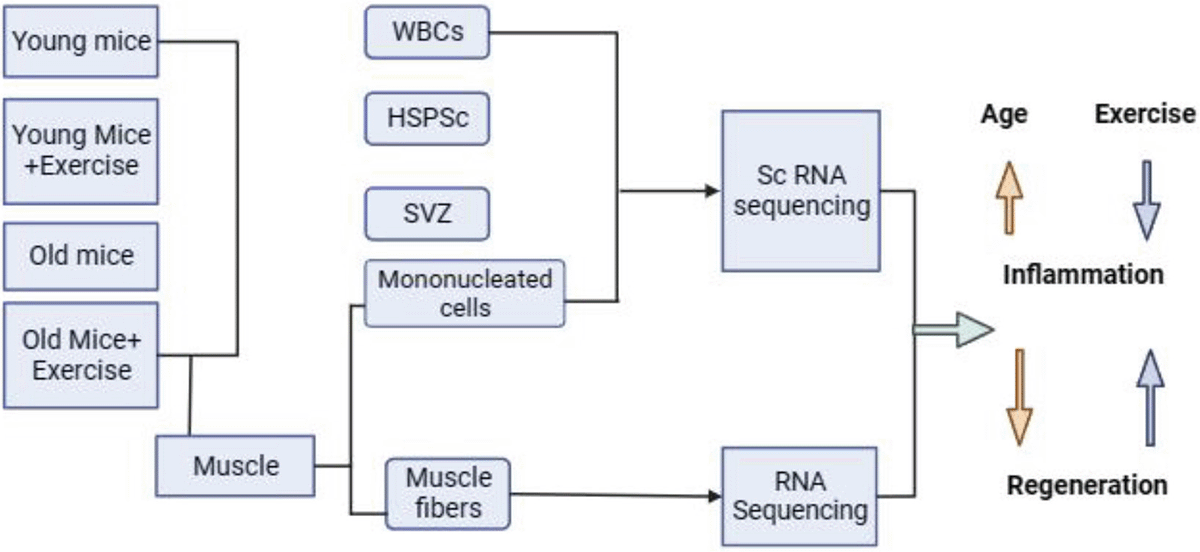
An extensive examination of 435,628 single cells was conducted across various tissues, including the SVZ of the brain, HSPCs in bone marrow, immune cells in peripheral blood, and skeletal muscle. This study involved both 4-month-old young mice and 22-month-old mice, investigating individuals with and without a voluntary exercise regimen. Mice were individually placed in cages with 12.7-centimetre-diameter wheels fitted with optical rotation sensors for 5 weeks During the period of voluntary wheel running. The control group, comprising non-exercised mice, was also individually housed in identical cages but lacked wheels.
A range of techniques was employed to investigate the comprehensive single-cell atlas, aiming to assess how exercise influences both stem cell function and tissue equilibrium in the context of aging.
1. Cell isolation
2. ELISA
3. Single-cell RNA sequencing
4. scRNA-seq data processing
5. RNA-Seq
6. Single Fiber isolation
7. RT-qPCR
8. Analysis of muscle regeneration
Results
1. The investigation aimed to assess the impact of aerobic exercise on the transcriptional profiles of stem cells and their respective niche cells in the neural, hematopoietic and muscular systems, of both young (4 months old) and old (22 months old) male mice. Following 5 weeks of running, tissues from control and exercised mice, including bone marrow, skeletal muscle, SVZ, brain, and blood, were collected. The study revealed that NSCs and neuroblasts experienced the most notable decline with age, and exercise increased the populations of these two cell types in both young and old mice. In the HSC compartment, aging correlated with an expansion of long-term HSCs (LT-HSCs) and a shift in cell expansion towards myeloid multipotent progenitors (MyMPPs).
2. Identifying differentially expressed genes (DEGs) among 22 primary cell types in Y-Ex, O-Ex, O-C, Y-C, and mice aimed to unveil shared and distinct gene expression modifications associated with exercise and aging. The results indicate that aging induces changes in the quantity and relative ratio of stem cell subsets and exercise-related genes (DEGs) in the brain, skeletal muscle, and the haematological system. Significantly, the most pronounced age-DEGs are present in monocytes, particularly within the blood. The SVZ exhibits a notable prevalence of common age-DEGs, as demonstrated by contrasting age-DEGs that are increased and downregulated in HSCs and the main immune cell types found in muscle, the SVZ and peripheral blood. Moreover, upregulated age-DEGs across cell types suggest an escalated inflammatory systemic environment in aging, as indicated by gene set enrichment analysis (GSEA). Increased activation and tumour necrosis factor-alpha (TNF-α) signalling and interleukin 6 (IL-6), IL-2 is noticeable in muscle and various cell types in peripheral blood and bone marrow, but this pattern is not observed in other cells of the subventricular zone (SVZ).
3. Exercise-induced alterations in gene expression, or exercise-DEGs, were generally less noticeable in old mice, except immune cells in the muscle, where significant impacts were seen. Exercise-associated activation of metabolic genes was identified by pathway analysis in young mice, especially in the SVZ, and was reduced in older animals. Furthermore, exercise inhibited multiple cell types’ TNF-α and other cytokine signalling pathways. Looking at age-related differentially expressed genes (age-DEGs), exercise restored expression of genes that are downregulated as people age, or “restored genes.” Inflammatory scores revealed a correlation between exercise and a reduced state of inflammation in most cell types, especially in young mice, suggesting that stem cells and their microenvironment are broadly protected against inflammation.
4. Investigating the influence of physical activity on inflammatory reactions in older animals, the research specifically centres on macrophages and monocytes originating from MyMPPs, which are the descendants of LT-HSCs.The analysis traces a path from LT-HSCs to muscle-specific clusters, revealing 20 clusters in monocytes/macrophages from muscle and blood. Notably, exercise demonstrates a favourable influence on the terminally differentiated muscle monocyte/macrophage population. Genes such as Pf4, C1qa, and CD206 are associated with a macrophage class characterized by phagocytic and tissue-remodelling traits, and these macrophages contain cytokines that have anti-inflammatory properties. Conversely, the functional study indicates that exercise increases the number of muscle-resident monocytes resembling M2 macrophages in the blood, whereas aging may decrease this subgroup, thereby altering the local stem cell environment.
5. To study cell-cell communication networks of skeletal muscle in young mice, scRNA-seq and CellChat analysis were employed. A partial recovery of half of the 38 signalling pathways—including those triggered by monocytes and macrophages—was observed after exercise in response to aging. Modified expression and multiple macrophages expressing these ligands were linked to changes in ANXA1, OSM, IL-1, and GAS signalling. Exercise correlated with increased Axl expression in MuSCs, suggesting potential benefits for exercise-induced self-renewal of MuSCs in older mice. Overall, these results point to the possibility that exercise revitalizes intercellular connections in the MuSC niche by immune-modulating skeletal muscle.
6. A study of cell-cell interactions in the SVZ revealed 52 signalling pathways in young mice, including six ECM-mediated and 52 involving secreted or cell surface chemicals. Mural cells participated in extracellular matrix (ECM) interactions, whereas OPCs contributed substantially to secreted and cell surface contacts. A beneficial impact on the communication network is suggested by the enhanced contacts observed during exercise between aNSCs, neuroblasts, and other SVZ cells. 27 signalling pathways were impacted by aging, and 13 of them partially recovered with exercise. Exercise restored cytokine signalling pathways, such as TWEAK signalling and TNFSF12 signalling linked to angiogenesis. All things considered; these results shed light on plausible processes by which physical activity improves brain function by rewiring cell-to-cell connections in the neurogenesis area.
7. RNA-Seq study of isolated muscle fibres reveals different transcriptional patterns in control fibres from young and old mice. Notably, exercise highlighted similarities in gene expression by inducing comparable transcriptome alterations in both age groups. SPP1 is a major myokine that is regulated by exercise, according to ligand-receptor interaction studies. Its expression is linked to modifications in target genes in different types of muscle cells, including proteins that regulate the immune system. ELISA results verified that older animals had lower Spp1 levels, which rose with exercise. Functional studies revealed that in aged mice, the synthesis of SPP1 induced by exercise enhanced the regeneration through immune system modulation and homeostasis of skeletal muscle and.
Discussion
The importance of a single-cell transcriptomic atlas lies in its coverage of 3 different categories of stem cells, their progeny, and neighbouring niche cells, obtained from both aged and young mice, with a specific emphasis on examining the effects of voluntary physical activity. The emphasis is on chronic inflammation associated with aging, marked by heightened expression of genes responsive to TNF-α and IFN-γ in their milieu’s cells and stem cells. Exercise is identified as a means to mitigate this inflammation, particularly in the brain, blood stem domains muscle, manifesting an anti-inflammatory influence. Notably, exercise-induced alterations in the MuSC compartment involve modifications in myofibers and FAPs, indicating crucial changes in niche cells that mediate beneficial effects on MuSCs in elderly animals. In the SVZ, there is a sustained increase in IFN signalling, potentially influenced by infiltrating T cells. Exercise is recognized for promoting the production of CX3CL1, known for its neuroprotective properties. This suggests that exercise may play a role in reducing neurotoxicity and alleviating neurodegenerative conditions (Figure 1). The comprehensive single-cell atlas and myofiber profiles offer valuable insights into aging, exercise-induced changes, and potential targets for treating age-related disorders, contributing to the extension of lifespan.

Figure 1: Schematic representation of the effect of exercise on aging.
References
1. Brunet A, Goodell MA, Rando TA. Ageing and rejuvenation of tissue stem cells and their niches. Nat Rev Mol Cell Biol. 2023;24(1):45–62.
2. Dulken BW, Buckley MT, Negredo PN, Cayrol R, Leeman DS, George BM, et al. niches. 2020;571(7764):205–10.
3. Ibrayeva A, Bay M, Pu E, Jörg DJ, Peng L, Jun H, et al. Early stem cell aging in the mature brain. Cell Stem Cell. 2021;28(5):955–966.e7.
4. Kalamakis G, Brüne D, Ravichandran S, Bolz J, Fan W, Ziebell F, et al. Quiescence Modulates Stem Cell Maintenance and Regenerative Capacity in the Aging Brain. Cell. 2019;176(6):1407–1419.e14.
5. Artegiani B, Lyubimova A, Muraro M, van Es JH, van Oudenaarden A, Clevers H. A Single-Cell RNA Sequencing Study Reveals Cellular and Molecular Dynamics of the Hippocampal Neurogenic Niche. Cell Rep. 2017;21(11):3271–84.
6. Liu L, Kim S, Buckley MT, Reyes JM, Kang J, Tian L, et al. Exercise reprograms the inflammatory landscape of multiple stem cell compartments during mammalian aging. Cell Stem Cell. 2023;30(5):689–705.e4.